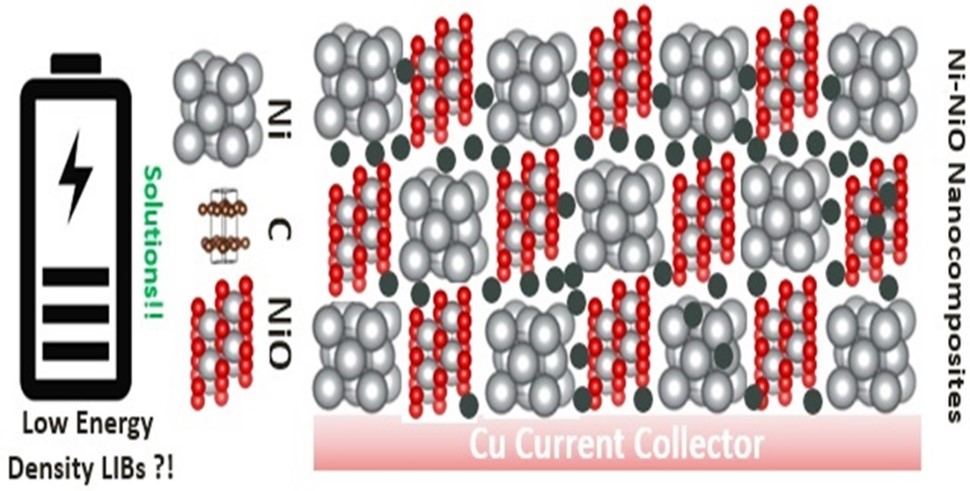
Microstructured pebble stone like Ni-NiO composite as anode of high-performance lithium-ion batteries
Downloads
Ni-NiO electrodes were synthesized via thermal oxidation of pure nickel powder and evaluated as anode of lithium-ion batteries (LIBs). The composite synthesized at 600˚C, 800˚C, and 1000˚C exhibited nanochips, crushed gravel stone, and pebble stone-like morphology, respectively. The nanochips- and crushed gravel stone featured-like electrodes exhibited erratic behavior, and specific capacity faded rapidly from 754.49 mAh g-1 and 101.12 mAh g-1 to 464.04 mAh g-1 and 9.55 mAh g-1, respectively over 10th cycle at a current rate of 1C as the electrode experiences internal short circuit. The pebble stone-like Ni-NiO electrode exhibited improved and stable cyclic performance with 1st discharge capacity of 365.17 mAh g-1 and reduced to 67.42 mAh g-1 even after 40th cycle at 1C current rate. The improved electrochemical performance of composite Ni-NiO with a pebble stone-like feature can be attributed to the mechanical stability of the electrode, which can buffer volume expansion, and the presence of more nanoparticles on the electrode surface allows more interaction with Li+.
Zhang J, Terrones M, Park CR, Mukherjee R, Monthioux M, Koratkar N, et al. Carbon science in 2016: Status, challenges and perspectives. Vol. 98, Carbon. Elsevier Ltd; 2016. p. 708–32.
Chemistry of Materials Editorial. Available from: https://doi.org/10.1021/acs.chemmater.
Rodrigues MTF, Babu G, Gullapalli H, Kalaga K, Sayed FN, Kato K, et al. A materials perspective on Li-ion batteries at extreme temperatures. Nat Energy. 2017 Aug;2(8).
Rahman MdA, Kim J, Hossain S. Recent advances of energy storage technologies for grid: A comprehensive review. Energy Storage. 2022 Jan 10;
Nardo M, Saisana M, Saltelli A, Tarantola S, Hoffman H, Giovannini E. Handbook on constructing composite indicators: methodology and user guide. Organisation for Economic Cooperation and Development (OECD). Statistics Working Paper JT00188147, OECD, France. 2005;
Winter M, Besenhard JO, Spahr ME, Novµk P. Insertion Electrode Materials for Rechargeable Lithium Batteries. https://doi.org/10.1002/(SICI)1521-4095(199807)10:10<725::AID-ADMA725>3.0.CO;2-Z
Van Schalkwijk WA, Scrosati B. Advances in Lithium-ion Batteries Kluwer Academic. Plenum, New York; 2002.
Ma D, Cao Z, Hu A. Si-based anode materials for li-ion batteries: A mini review. Vol. 6, Nano-Micro Letters. Shanghai Jiaotong University; 2014. p. 347–58.
An Q, Lv F, Liu Q, Han C, Zhao K, Sheng J, et al. Amorphous vanadium oxide matrixes supporting hierarchical porous Fe3O4/graphene nanowires as a high-rate lithium storage anode. Nano Lett. 2014 Nov 12;14(11):6250–6.
Dai R, Wang Y, Da P, Wu H, Xu M, Zheng G. Indirect growth of mesoporous Bi@C core-shell nanowires for enhanced lithium-ion storage. Nanoscale. 2014 Nov 7;6(21):13236–41.
Yang C, Jiang Y, Liu X, Zhong X, Yu Y. Germanium encapsulated in sulfur and nitrogen co-doped 3D porous carbon as an ultra-long-cycle life anode for lithium ion batteries. J Mater Chem A Mater. 2016;4(48):18711–6.
Choi JW, Aurbach D. Promise and reality of post-lithium-ion batteries with high energy densities. Vol. 1, Nature Reviews Materials. Nature Publishing Group; 2016.
Chan CK, Ruffo R, Hong SS, Cui Y. Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes. J Power Sources. 2009;189(2):1132–40.
Ruffo R, Hong SS, Chan CK, Huggins RA, Cui Y. Impedance analysis of silicon nanowire lithium ion battery anodes. The Journal of Physical Chemistry C. 2009;113(26):11390–8.
Li, Si. 648 wileyonlinelibrary.com [Internet]. 2015. Available from: www.MaterialsViews.com
Ferg E, Gummow RJ d, De Kock A, Thackeray MM. Spinel anodes for lithium‐ion batteries. J Electrochem Soc. 1994;141(11):L147.
Zhao Y, Li X, Yan B, Xiong D, Li D, Lawes S, et al. Recent Developments and Understanding of Novel Mixed Transition-Metal Oxides as Anodes in Lithium Ion Batteries. Vol. 6, Advanced Energy Materials. Wiley-VCH Verlag; 2016.
Li T, Li X, Wang Z, Guo H, Li Y, Wang J. A new design concept for preparing nickel-foam-supported metal oxide microspheres with superior electrochemical properties. J Mater Chem A Mater. 2017;5(26):13469–74.
Hao L, Liang M, Weiwei S, Yong W. Bimetal-Organic Framework: One-Step Homogenous Formation and its Derived Mesoporous Ternary Metal Oxide Nanorod for High-Capacity, High-Rate, and Long-Cycle-Life Lithium Storage. Adv Funct Mater. 2016 Feb 16;26(7):1098–103.
Chen Z, Zhou M, Cao Y, Ai X, Yang H, Liu J. In situ generation of few-layer graphene coatings on SnO 2-SiC core-shell nanoparticles for high-performance lithium-ion storage. Adv Energy Mater. 2012 Jan;2(1):95–102.
Liu H, Wang G, Liu J, Qiao S, Ahn H. Highly ordered mesoporous NiO anode material for lithium ion batteries with an excellent electrochemical performance. J Mater Chem. 2011 Mar 7;21(9):3046–52.
Rahman MA, Wong YC, Song G, Zhu DM, Wen C. Improvement on electrochemical performances of nanoporous titania as anode of lithium-ion batteries through annealing of pure titanium foils. Journal of Energy Chemistry. 2018 Jan 1;27(1):250–63.
Rahman MA, Wen C. Nanogravel structured NiO/Ni foam as electrode for high-performance lithium-ion batteries. Ionics (Kiel). 2015 Oct 22;21(10):2709–23.
Zhu Y, Choi SH, Fan X, Shin J, Ma Z, Zachariah MR, et al. Recent Progress on Spray Pyrolysis for High Performance Electrode Materials in Lithium and Sodium Rechargeable Batteries. Vol. 7, Advanced Energy Materials. Wiley-VCH Verlag; 2017.
Cai Z, Xu L, Yan M, Han C, He L, Mulonda Hercule K, et al. Manganese Oxide/Carbon Yolk-Shell Nanorod Anodes for High Capacity Lithium Batteries [Internet]. 2014. Available from: http://pubs.acs.org
Wang Y, Xu J, Wu H, Xu M, Peng Z, Zheng G. Hierarchical SnO 2-Fe 2O 3 heterostructures as lithium-ion battery anodes. J Mater Chem. 2012 Nov 7;22(41):21923–7.
Rahman MA, Wong YC, Song G, Wen C. A review on porous negative electrodes for high performance lithium-ion batteries. Journal of Porous Materials. 2015 Oct 7;22(5):1313–43.
Sun MH, Huang SZ, Chen LH, Li Y, Yang XY, Yuan ZY, et al. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem Soc Rev. 2016 Jun 21;45(12):3479–563.
Zheng M, Tang H, Li L, Hu Q, Zhang L, Xue H, et al. Hierarchically nanostructured transition metal oxides for lithium‐ion batteries. Advanced Science. 2018;5(3):1700592.
Kumar Rai A, Tuan Anh L, Park CJ, Kim J. Electrochemical study of NiO nanoparticles electrode for application in rechargeable lithium-ion batteries. Ceram Int. 2013 Aug;39(6):6611–8.
Li X, Dhanabalan A, Wang C. Enhanced electrochemical performance of porous NiO-Ni nanocomposite anode for lithium ion batteries. J Power Sources. 2011 Nov 15;196(22):9625–30.
Rahman MA, Zhu X, Wen C. Fabrication of Nanoporous Ni by Chemical Dealloying Al from Ni-Al Alloys for Lithium-ion Batteries [Internet]. Vol. 10, Int. J. Electrochem. Sci. 2015. Available from: www.electrochemsci.org
Wang C, Wang D, Wang Q, Chen H. Fabrication and lithium storage performance of three-dimensional porous NiO as anode for lithium-ion battery. J Power Sources. 2010 Nov 1;195(21):7432–7.
Kim GP, Park S, Nam I, Park J, Yi J. Synthesis of porous NiO materials with preferentially oriented crystalline structures with enhanced stability as lithium ion battery anodes. J Power Sources. 2013;237:172–7.
Du M, Li Q, Pang H. Oxalate-derived porous prismatic nickel/nickel oxide nanocomposites toward lithium-ion battery. J Colloid Interface Sci. 2020 Nov 15;580:614–22.
Singh J, Lee S, Kim S, Singh SP, Kim J, Rai AK. Fabrication of 1D mesoporous NiO nano-rods as high capacity and long-life anode material for lithium ion batteries. J Alloys Compd. 2021 Jan 5;850.
Duraisamy E, Sujithkrishnan E, Kannadasan K, Prabunathan P, Elumalai P. Facile metal complex-derived Ni/NiO/Carbon composite as anode material for Lithium-ion battery. Journal of Electroanalytical Chemistry. 2021 Apr 15;887.
Cheng CF, Chen YM, Zou F, Yang KC, Lin TY, Liu K, et al. Nanoporous gyroid Ni/NiO/C nanocomposites from block copolymer templates with high capacity and stability for lithium storage. J Mater Chem A Mater. 2018;6(28):13676–84.
Liu L, Li Y, Yuan S, Ge M, Ren M, Sun C, et al. Nanosheet-based NiO microspheres: Controlled solvothermal synthesis and Lithium storage performances. Journal of Physical Chemistry C. 2010 Jan 14;114(1):251–5.
Jae W, Song J, Hong JJ, Kim J. Raspberry-like hollow Ni/NiO nanospheres anchored on graphitic carbon sheets as anode material for lithium-ion batteries. J Alloys Compd. 2019 Oct 15;805:957–66.
Varghese B, Reddy M V., Yanwu Z, Lit CS, Hoong TC, Rao GVS, et al. Fabrication of NiO nanowall electrodes for high performance lithium ion battery. Chemistry of Materials. 2008 May 27;20(10):3360–7.
Bell J, Ye R, Ahmed K, Liu C, Ozkan M, Ozkan CS. Free-standing Ni-NiO nanofiber cloth anode for high capacity and high rate Li-ion batteries. Nano Energy. 2015 Nov 1;18:47–56.
Liu L, Guo Y, Wang Y, Yang X, Wang S, Guo H. Hollow NiO nanotubes synthesized by bio-templates as the high performance anode materials of lithium-ion batteries. Electrochim Acta. 2013;114:42–7.
Wu MS, Lin YP. Monodispersed macroporous architecture of nickel-oxide film as an anode material for thin-film lithium-ion batteries. Electrochim Acta. 2011 Feb 1;56(5):2068–73.
Su D, Kim HS, Kim WS, Wang G. Mesoporous nickel oxide nanowires: Hydrothermal synthesis, characterisation and applications for lithium-ion batteries and supercapacitors with superior performance. Chemistry - A European Journal. 2012 Jun 25;18(26):8224–9.
Pang Y, Zhang J, Chen D, Jiao X. 3D hierarchical porous NiO nanoflowers as an advanced anode material with remarkable lithium storage performance. RSC Adv. 2016;6(36):30395–400.
Jadhav HS, Thorat GM, Mun J, Seo JG. Self-assembled hierarchical 3D - NiO microspheres with ultra-thin porous nanoflakes for lithium-ion batteries. J Power Sources. 2016 Jan 20;302:13–21.
Yuan YF, Xia XH, Wu JB, Yang JL, Chen YB, Guo SY. Hierarchically ordered porous nickel oxide array film with enhanced electrochemical properties for lithium ion batteries. Electrochem commun. 2010 Jul;12(7):890–3.
Zhu X, Luo B, Butburee T, Zhu J, Han S, Wang L. Hierarchical macro/mesoporous NiO as stable and fast-charging anode materials for lithium-ion batteries. Microporous and Mesoporous Materials. 2017 Jan 15;238:78–83.
Li Q, Yi Z, Cheng Y, Wang XX, Yin D, Wang L. Microwave-assisted synthesis of the sandwich-like porous Al 2 O 3 /RGO nanosheets anchoring NiO nanocomposite as anode materials for lithium-ion batteries. Appl Surf Sci. 2018 Jan 1;427:354–62.
Wang X, Yang Z, Sun X, Li X, Wang D, Wang P, et al. NiO nanocone array electrode with high capacity and rate capability for Li-ion batteries. J Mater Chem. 2011 Jul 21;21(27):9988–90.
Li T, Ni S, Lv X, Yang X, Duan S. Preparation of NiO-Ni/natural graphite composite anode for lithium ion batteries. J Alloys Compd. 2013 Mar 15;553:167–71.
Zhu XJ, Hu J, Dai HL, Ding L, Jiang L. Reduced graphene oxide and nanosheet-based nickel oxide microsphere composite as an anode material for lithium ion battery. Electrochim Acta. 2012 Mar 1;64:23–8.
Mai YJ, Tu JP, Gu CD, Wang XL. Graphene anchored with nickel nanoparticles as a high-performance anode material for lithium ion batteries. J Power Sources. 2012 Jul 1;209:1–6.
Qiu Y, Huang H, Song W, Gan Y, Wang K, Zhang J, et al. In-situ electrolytic synthesis and superior lithium storage capability of Ni–NiO/C nanocomposite by sacrificial nickel anode in molten carbonates. J Alloys Compd. 2020 Sep 5;834.
Huang XH, Tu JP, Xia XH, Wang XL, Xiang JY, Zhang L. Porous NiO/poly(3,4-ethylenedioxythiophene) films as anode materials for lithium ion batteries. J Power Sources. 2010 Feb 15;195(4):1207–10.
Huang XH, Tu JP, Xia XH, Wang XL, Xiang JY. Nickel foam-supported porous NiO/polyaniline film as anode for lithium ion batteries. Electrochem commun. 2008 Sep;10(9):1288–90.
Rahman MdA, Rahman MdM, Song G. A review on binder‐free NiO‐Ni foam as anode of high performance lithium‐ion batteries . Energy Storage. 2021 Aug 13;
Sarker B, Rahman MA, Rahman MM, Islam MS. Fabrication of ni-nio foams by powder metallurgy technique and study of bulk crushing strength. Trends in Sciences. 2021 Dec 1;18(23).
Cabanas-Polo S, Bermejo R, Ferrari B, Sanchez-Herencia AJ. Ni-NiO composites obtained by controlled oxidation of green compacts. Corros Sci. 2012 Feb;55:172–9.
Xia Q, Zhao H, Teng Y, Du Z, Wang J, Zhang T. Synthesis of NiO/Ni nanocomposite anode material for high rate lithium-ion batteries. Mater Lett. 2015 Mar 1;142:67–70.
Huang XH, Tu JP, Zhang CQ, Xiang JY. Net-structured NiO–C nanocomposite as Li-intercalation electrode material. Electrochem commun [Internet]. 2007;9(5):1180–4. Available from: https://www.sciencedirect.com/science/article/pii/S1388248107000185
Zhu H, Zeng X, Han T, Li X, Zhu S, Sun B, et al. A nickel oxide nanoflakes/reduced graphene oxide composite and its high-performance lithium-storage properties. Journal of Solid State Electrochemistry. 2019 Jul 19;23(7):2173–80.
Xu C, Sun J, Gao L. Large scale synthesis of nickel oxide/multiwalled carbon nanotube composites by direct thermal decomposition and their lithium storage properties. J Power Sources. 2011 Jun 1;196(11):5138–42.
Yu Y, Gu L, Zhu C, Tsukimoto S, Van Aken PA, Maier J. Reversible storage of lithium in silver‐coated three‐dimensional macroporous silicon. Advanced materials. 2010;22(20):2247–50.
Huang XH, Tu JP, Zhang B, Zhang CQ, Li Y, Yuan YF, et al. Electrochemical properties of NiO-Ni nanocomposite as anode material for lithium ion batteries. J Power Sources. 2006 Oct 20;161(1):541–4.
Ni S, Li T, Lv X, Yang X, Zhang L. Designed constitution of NiO/Ni nanostructured electrode for high performance lithium ion battery. Electrochim Acta. 2013 Feb 28;91:267–74.
Oh SW, Bang HJ, Bae YC, Sun YK. Effect of calcination temperature on morphology, crystallinity and electrochemical properties of nano-crystalline metal oxides (Co3O4, CuO, and NiO) prepared via ultrasonic spray pyrolysis. J Power Sources. 2007;173(1):502–9.
Werber T. Joining of nickel powder grains by thermal oxidation. Vol. 42, Solid State Ionics. 1990. https://doi.org/10.1016/0167-2738(90)90009-G
Hernández N, Moreno R, Sánchez-Herencia AJ, Fierro JLG. Surface behavior of nickel powders in aqueous suspensions. J Phys Chem B. 2005;109(10):4470–4.
Evans HE. Stress effects in high temperature oxidation of metals. International materials reviews. 1995;40(1):1–40.
Clarke DR. S tress generation during high-temperature oxidation of metallic alloys. Vol. 6, Current Opinion in Solid State and Materials Science. 2002.
Huang Y, Sun W, Xu K, Zhang J, Zhang H, Li J, et al. Robust interphase on both anode and cathode enables stable aqueous lithium-ion battery with coulombic efficiency exceeding 99%. Energy Storage Mater. 2022;46:577–82.
Wang Z, Zhang P, Li J, Zhang C, Jiang JX, Lv M, et al. A low-cost naphthaldiimide based organic cathode for rechargeable lithium-ion batteries. Front Chem. 2022;10.
Yasin G, Arif M, Ma J, Ibraheem S, Yu D, Zhang L, et al. Self-templating synthesis of heteroatom-doped large-scalable carbon anodes for high-performance lithium-ion batteries. Inorg Chem Front. 2022;9(6):1058–69.
Lee SH, Harding JR, Liu DS, D’Arcy JM, Shao-Horn Y, Hammond PT. Li-anode protective layers for Li rechargeable batteries via layer-by-layer approaches. Chemistry of Materials. 2014;26(8):2579–85.
Zhao W, Luo G, Wang CY. Modeling nail penetration process in large-format Li-ion cells. J Electrochem Soc. 2014;162(1): A207.
Rahman MA, Wen C. A study of the capacity fade of porous NiO/Ni foam as negative electrode for lithium-ion batteries. Ionics (Kiel). 2016 Feb 1;22(2):173–84.
Maleki H, Howard JN. Internal short circuit in Li-ion cells. J Power Sources. 2009;191(2):568–74.
Cai W, Wang H, Maleki H, Howard J, Lara-Curzio E. Experimental simulation of internal short circuit in Li-ion and Li-ion-polymer cells. J Power Sources. 2011;196(18):7779–83.
Li B, Zhang H, Wang D, Lv H, Zhang C. Agricultural waste-derived activated carbon for high performance lithium-ion capacitors. RSC Adv. 2017;7(60):37923–8.
Hosono E, Matsuda H, Honma I, Ichihara M, Zhou H. High-Rate Lithium Ion Batteries with Flat Plateau Based on Self-Nanoporous Structure of Tin Electrode. J Electrochem Soc. 2007;154(2): A146.
Huang XH, Tu JP, Zhang CQ, Zhou F. Hollow microspheres of NiO as anode materials for lithium-ion batteries. Electrochim Acta. 2010 Dec 1;55(28):8981–5.
Poizot P, Laruelle S, Grugeon S, Dupont L, Tarascon JM. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature. 2000;407(6803):496–9.
Jin J, Wu L, Huang S, Yan M, Wang H, Chen L, et al. Hierarchy Design in Metal Oxides as Anodes for Advanced Lithium‐Ion Batteries. Small Methods. 2018;2(11):1800171.
Nie M, Chalasani D, Abraham DP, Chen Y, Bose A, Lucht BL. Lithium ion battery graphite solid electrolyte interphase revealed by microscopy and spectroscopy. The Journal of Physical Chemistry C. 2013;117(3):1257–67.
Aurbach D, Ein‐Eli Y, Markovsky B, Zaban A, Luski S, Carmeli Y, et al. The study of electrolyte solutions based on ethylene and diethyl carbonates for rechargeable Li batteries: II. Graphite electrodes. J Electrochem Soc. 1995;142(9):2882.
Lu M, Cheng H, Yang Y. A comparison of solid electrolyte interphase (SEI) on the artificial graphite anode of the aged and cycled commercial lithium ion cells. Electrochim Acta. 2008;53(9):3539–46.